U.S., BioWorld MedTech
2023 FDLI Enforcement Conference
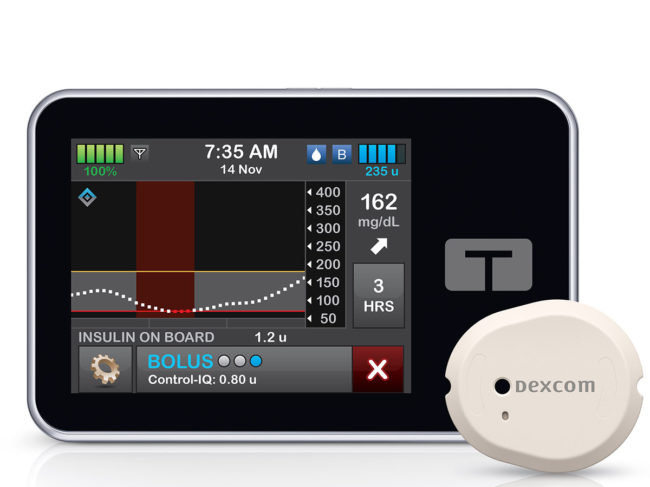
FDA facing a ‘somewhat unimaginable’ volume of applications for LDTs
Read More2023 FDLI Enforcement Conference
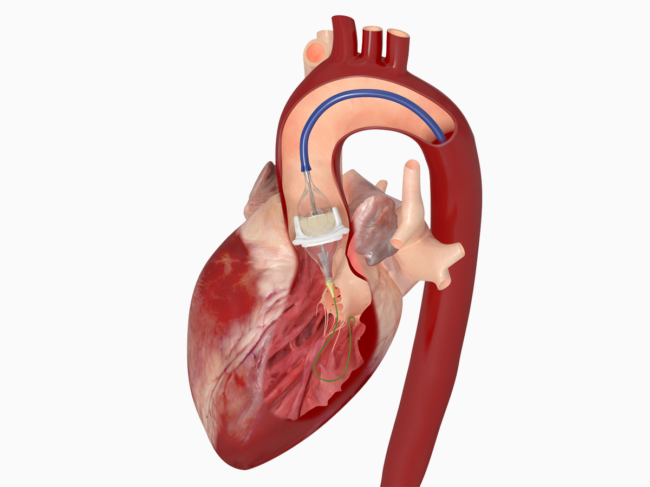
Abiomed warning letter for final deviation seen as opening salvo
Read More2023 FDLI Enforcement Conference